?��ǰ����“Ԭ������”ר��ƾ֤�ѽ�������������������PK����Ҫ����������Դ������Ԭ������ | ����������������ۣ�ʮ��_�ϣ���LBA��������˫����������ҩ����ս�����������ڽ������������������ص����ذ����������ش�ҩ����ӵ�δ���������������Լ�Զ���ȷ������̽��������
“Ԭ������”ר��ϵ������2��������¼���ҽҩ�Ź��ںŴ���Ŀ���ѧ��ר���������ݾ�Ϊ��2��������¼���ҽҩҩ���о����������ѧ����Ԭ�Dz�ʿԭ��������
����������Case studies��
���°����о���Ϊ��˵������˫�����Է��Ӷ���������������������˼����������������ҩ�↑���IJ�����ƺ�ʵ�鶨������������PK����������
��������1���ڷ��ٴ��о��ж����ܺ�����˫������ҩ�ﲢ����ADA��PK��Ӱ��������
������X��һ�ֻ���֧�ܵ��������ϸ�����ӵ�˫�����Կ�����������Դ������ע�������������֧�ܲ�Ʒƽ̨�����ڷ��ٴ������и�Ƶ�ʵ��յ�����ԭ��������Ϊ�˸��õ�ڹ��ADAs����ʱ�Ķ���ѧ����������������С����鿪�����ֲ���PKҪ�����ⶨ����Ѫ���е���ҩ�������ҩ���Ũ����������PKҪ����ʹ�����ֿ����֧�ܵĿ�ܾ�������������������PKҪ����ʹ��һ�ְ���ϸ��������Ϊ�����Լ�������һ����Ϊ����Լ�������������Ӳ쵽����ʹ������PKҪ��������ͬ����Ʒ������Ũ����ʱ���ϵ��Щ��ȫ��ͬ���������ڽ�����ʱ���֮������ҩ��Ũ�Ⱥ��ǵ͵�ʱ������������ҩ��Ũ���Ե�����ҩ��Ũ�ȵ�ʱ��������
����Щʱ����Ӳ쵽�ĵ�Ũ�Ȳ��ֻ�������������������������������ADA������ת����biotransformation������Ϊ������ܵĻ���Ե��ԭ�������������һ���о���ȷ�����ֲ��Ļ���Ե��ԭ������������ת���յ��IJ��ȹ��������б����ţ�target interference����ADA����������Ϊ����֤����˫�����Է��ӵ���������ת���������������Ѱ���ˮ���Ƿ�չ����Ӱ���ȹ��ԵĽṹȱ�������ʿ��������������Ž�����(ligand-binding mass spectrometry����LBMS)Ҫ������ʹ�ÿ���Fc�����Ͳ���Ͱ�Ŀ�IJ�������Ȼ����з���������������������������ESI-MS���Ž������Һ��ɫ���ԷǶ��ִ�л��Ʒ�����˸��������ʵ���ɢ���ж�������
������ʾ��������ת����Ч��Ϊ����������ADA������ע˫�����Է��ӱ���ĸ��ҩ���ADA����Ƶ�ʸ���������ֻ�����ͨ������ԭ�Ա�������֤ʵ���ADAs�����к���ADAs����������ADAs�����ڿ��ٵ�ɨ������ҩ����������Щ����Ҳ����ڹ��Ϊ��ҩ��Ũ���㹻��ʱ������Դ��ϸ������ռ�����ض��IJ���/���λ������������б�����������Ч����ע������ҩ�������ڽṹ�ȹ�������PK���ݲ��������ҩ��IJ��ȹ�����������������������ADAs��ˮƽ����ɨ��ҩ������ʸ���������
��������2��һ��F(ab’)2����������ת��Ϊ��������F(ab)�����PK����������
�������о��漰һ��˫������F(ab’)2������һ��anti-VEGF arm��һ��anti-Ang2 arm����������ڲ�������ע�䣨intravitreal administration����������Ĥ���Լ���������ʪ�������Իư߱��Ժ������Իư�ˮ���������ݱ����������F(ab’)2�����������������ǰ�������Դ�Կ���(PEA)�����ںܴ��������Ⱥ����������δ����ҩ���ʳз�����Ѫ��������������֤ʵ����һ���������Ƴ���ЩԤ�ȱ������Դ�Կ���Ľ�����λ��hinge epitopes��ʹ�ø÷�����ֻ�е��������������fab�Ž���һ��������
�������ע���벣���壨�京��glutathione��Ϊ��������Ʒ��һ�����������ܾ�״��������ԣ�������ҩ���������ת��Ϊindividual Fabs�������ɨ����(t1/2 <1��)����ͨ�����ò������Fab (t1/2 Լ3��)��F(ab’)2 (t1/2 Լ3��)��ɨ����Ҫ��(δ�������Ӳ�Ч��)������
��ע��һ��ƽ������������t1/2��ɨ���ʵĹ�ϵ������������˥�ڻ�ȡ����ҩ���������������Ǽ�����������㶨������t1/2��ɨ����֮��Ĺ�ϵ��ȷ���ɷ��ȵģ�

�������������F(ab’)2��individual Fab��Ƭ����������ǻ(�������ˮ��֮��)��ϵͳѭ��֮��������������Ԥ�ϵ����������ද������Ĥ����Ѫ��ģ�ӣ�rodent choroidal neovascularization models����ʾ����individual Fabs�������������������Ϊ����ȫ����ò����ҩ��̻¶����������������Ҫ������3��ҩ����ʽ���������������������֤��3�ֵ�����PK����Ҫ����������ǰ��ѧ��һ��˼����ʹ��һ����PK����Ҫ�����ⶨ������3����ʽ���������������÷�����һ����ӱ�ṹ����������Ҫ����������ת���Ķ���ѧ�����������ò�÷������ڵ���Ϊ�Ǻ���Ҫ��������
���������������ELISAҪ��ʹ���ο��ģ�immobilized�������Ѱ�Ang2�������������Ȼ�����biotin-VEGF����������streptavidin-HRP������һ��sequential sandwich������������Ӵ�����Ľ�����ʵ��֤ʵ���ֲ������ý��ܹ������Եؼ��F(ab’)2��������ⲻ������Fab�����е��κ�һ����������������Fab���ӵ�Ҫ�춼�������ƵĻ������ã�ʹ�ø��Եİб�-Ang2��б�-VEGF�����������в���Fabs������Ȼ�����biotin-sheep antihuman IgG������������(H&L)���������������streptavidin-HRP���м��������
ֵ��ע�ص����������ɲⶨFab����������Ҳ���Լ���������������ֻ��ʹ��Fabs��Ϊ��Ʒ��standards���ͱ���Ʒ��controls������������Fabʱ����Ҳ����������F(ab’)2�������������ʹ��F(ab’)2���κ�һ��Fab�Ļ�������������ͨ�����������ӣ��������ԣ��Ķ���Ч���м�ȥFabЧ����������һ��Fab������������֤��������Щ����Ҫ��������������Ѫ�������ķ��ٴ��о�����Ҳ��֤����ЩҪ�������ʳз���Ѫ���ˮ/������/����Ĥ����ȡ����ҩ��Ķ���������Ѫ�嶨����������һ����ս�Ǽ���ng/ml�������͵�ҩ��Ũ��������Ҳ�Dz�����ǻ����ҩ��ҩ��һ���ص㣺��ҩ����С(ͨ����<1 mg/eye)���ҩ���ڵִ�ϵͳѭ��ʱ���������˸߱�����ϡ��������
��������3���ڷ��ٴ��о��м������˫������ҩ������ת�����ܺͻ��ԣ�total and active��ҩ��Ķ���������
һ���������˳Ӧ֢��˫�����Ե���¡�����������ϸ����ò�Ŀ�ԭ������ҩ������ȡ���ڵ���¡����������Ž�ۣ�both binding arms��������������Ȼ����������������������������������������һ���Ž���з����˷��������(post-translational modification����PTM)������������������PTM���������ڲ�������������Ե�����������ͨ���Ѱ��ʹ��̶�ɨ�����������ڲ��ȹ̵İ������ϵĵ�ͻ�䣨point mutation������ȫ������б���Ž�������������������еڶ����Ž�۹�Ч��ҩ���������ۻ��������ܻ����ϻ���ҩ���Ž�ڶ����е������������ڶ�θ�ҩ���շ����Է�Ӧ������
�Ըõ�����������ת�������������о������ڱ��е�������ת���Ķ���ѧ��ˮƽ���������Ϊ��һ�������õ����ṩָ���������������ʹ������ӵ�Fc�����������Ž�IJ����Լ�������һ���Ž����õ���ҩ��PK����Ҫ��������֮��һ�ֻ���ҩ��PK����Ҫ�죺��Ȼ�ðб��Ѱ���ΪPTM-liable functional domain�IJ����Լ�����ʹ�ÿ���Fc�Լ���Ϊ����Լ�������ʹ��Ұ���ͺ�ӵ�е�ͻ���ҩ��Ļ���������������PKҪ����������ҩ�ﱣ��������������ܹ����ֺ�ȷ�ⶨ����ҩ��İٷֱ�������֮�����������˸�ҩ�������ʳз���ϵ�PK����������ʹ�����ܺͻ���PK����Ҫ�����ⶨ�о��������ܺͻ���ҩ���Ũ��������ʵ������֤ʵ����������Ʒ�е�ҩ�ﷺ����PTM�������һ���ҩ��Ũ�Ȱٷֱ���ʱ�����������������ڽ�ģ�ͷ��������������ٴ��о�����̭�����������������ҩ��Ũ�ȵĽ������������ٴ��о�Ч�������ھ���ʹ�û���PK����Ҫ����Ϊ֧���ٴ��о�����ҪҪ������������FIH�о���������PK����Ҫ������ڽ�һ����ò����ҩ������壨inactive drug variants��DZ�ڵ��ۻ������PK/PD�Ͷ��Ե�Ӱ��������
�ش�ҩ����ӵ�δ��������������
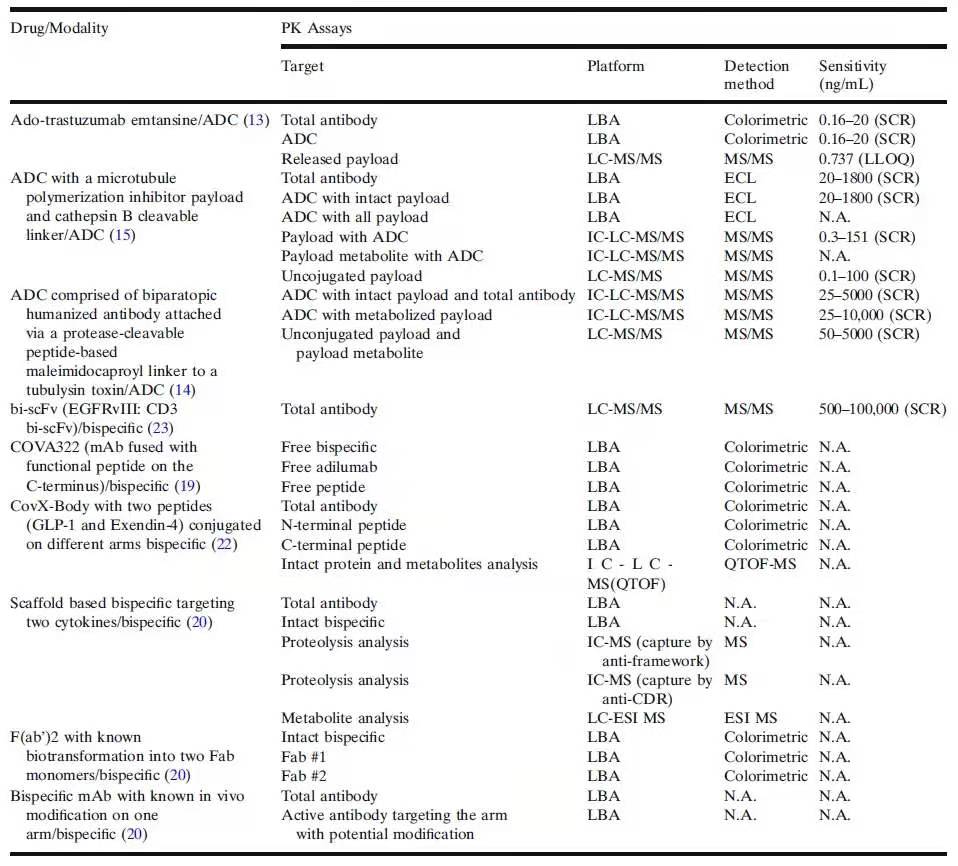
���1�����ܽ������һ��ƽ����Ҫ����PK����Ҫ���ADAҪ������ADC��˫�����Կ���Ļ���ҩ������ѧ������ԭ������������Ҫ����Ŀ�����������������硢���桢���䡢����������Ҫ����������ڹ����������ش�ļ縺��������ijЩ��������������������Ŀ�����������������ܾ��ж���������������������Ĺ�Ч���DZ�ڵ�����ת������������������Ҫ�����Ŀ��֪���������������ƺ���һ��ֱ�ӵĽ���ƻ�������“����ʽtwo-point”�Ž�Ҫ�죨�����ʽLBA�������ʺ��������ж���>3���ṹ�ķ���“������intactness”������ֻ�����߲���IC-��-LC-MS/MSҪ���������Ͽ���ʶ�����Զ����Ч����������������й�“������”��Ч����Ϣ��Ȼȱʧ������������������ش�ҩ��ģʽ��complex drug modalities��������ת��Խ��Խ�ܵ��Ĺ�ע��������LBAҪ��ͣ�IC-��LC-MS/MSҪ����������DZ�ڵ�����ת���ṩ�����Ϣ����������CovX-Body�ͻ��ڿ�ܣ�scaffold-based����˫�����Կ���İ�������ע����������PK��Ϊ����������´�����DZ������ת��������������������Ҫ�µ�����Ҫ������һ���о�������
��1.ADC��˫�����Կ����PK����Ҫ�죨SCR���������߹�ģ���ECL�����绯ѧ�������N.A.���������ã�
 ��ע�������вο����ı�ע������������
�������ʹ��multiplexed PK/ADAҪ���Բⶨ����ҩ����ֱ乹�壨variants������ص�ADA�Ǻ�����Ҫ������ͬʱ����Ҳ��DZ�ڵ�����ת���ṩ�����Ϣ������Multiplexed���Ҫ�������Ž��Ʒ���������Ӧ����������δ�������ش�ҩ��ģʽ“biotransformation ready”��multiplexed����Ҫ�������������������ش�ҩ�������������������������ת�����������Զ�ҩ��/ҩ��乹������ֵ����߷�Ӧ���������Ľ��������ݶ��ش�ҩ��ģʽ��ϣ�������������������գ�HR-MS�������������Ѱ��ʺ�ëϸ��Western Blot��������������
HR-MS �������������Ѱ���
��ע�������вο����ı�ע������������
�������ʹ��multiplexed PK/ADAҪ���Բⶨ����ҩ����ֱ乹�壨variants������ص�ADA�Ǻ�����Ҫ������ͬʱ����Ҳ��DZ�ڵ�����ת���ṩ�����Ϣ������Multiplexed���Ҫ�������Ž��Ʒ���������Ӧ����������δ�������ش�ҩ��ģʽ“biotransformation ready”��multiplexed����Ҫ�������������������ش�ҩ�������������������������ת�����������Զ�ҩ��/ҩ��乹������ֵ����߷�Ӧ���������Ľ��������ݶ��ش�ҩ��ģʽ��ϣ�������������������գ�HR-MS�������������Ѱ��ʺ�ëϸ��Western Blot��������������
HR-MS �������������Ѱ���
���������ҩ�������ij��ѡ�������LBA��LC-MS/MS����������������Ѱ��ʵĶ�������ּ�ڽ�һ���ش��ҩ�������Ϊһ���������о��������ܹ�չ����Ҫ�ĸ������ṹ������ת���������Ϣ�����������Ѱ�LC-MS����ͨ�������ε�������ת���о��Ķ�������������Murphy����ʹ��IC-LC Q-ToF MS������CovX-Body˫�����Կ����Ϸ������б����Ѱ�ø���е��ĺ���8����������Ķ�������He����ʹ�ø������ʵ�QTOF-MS��IC-LC���Ž������ֳɵ���С��Ѫ���������б������ɲ��DARs��ADC������ת���˵ı����壨biotransformed variants������������/ɾ����hexose, glutathione, cysteine�Լ�linker-drug������������������HR-MS��Ѹ�ٶȺ����������ʷ�������������ʹ���ڶ������������е����и��ձ��Ӧ��������
Jian���˽�����IC-LC-QTOF-MS������������������С��Ѫ��������mAbs�ľ��Զ������������������ޣ�LLOQ��Ϊ1000ng/mL��20 ?LѪ����Ʒ���������ɽ���250ng/mL��������ʹ��200?L��Ʒ���������Ż������ݴ��óͷ�ս�Ժ�����LLOQ����50ng/mL������ʹ������������������Jian�������չʾ��GLP1-Fc�ں��ѰĶ������ͬʱ�����ڶ�С����о�����������ʶ���˸�ҩ���������Ҫ�Ѱ�ø�����Ʒ������
Lanshoeft������֤�˻���multiplex IC-LC-HRMS��PK����Ҫ�����������ڷ��ٴ�PK�о�������Ҫ��ͬʱ���������˴���Ѫ������С��˽����IgG1���������Ƶ�����Jin�����ڴ���Ѫ���ж�����������trastuzumab emtansine��һ��ADC��������Ҫ��DAR���������䶨������ֵԼΪ20ng/mL�������Զ�̬��ģΪ5-100?g/mL������
��һ����Ȥ��Ӧ�������Zhang���˱����������ڷDZ���LC�����¶�native intact mAb���еĶ����������������Ϊʹ��LC-HR-MS������multiplex�ķ��������Žᣨbound��/δ�Žᣨunbound��PK����Ҫ���Լ��������ֹ�Ч��PK�Ķ���Ҫ���ṩ��ʱ��������
ëϸ��Western Blot��������Ҫ��
ëϸ��Western blot����Ҳ��Ϊëϸ���������߲ⶨ��capillary nanoimmunoassay����CNIA����������������Protein Simple(San Jose, CA)��ҵ����Simple Western system������ָëϸ�ܵ�Ӿ���߲ⶨϵͳ�������ṩ�����Ѱ��ʾ�ϸ�͵�ɵ���ɢ����������������ɢ�ͼ��֮ǰ���������Ž��IC-LC-MS���ղ������Western blot������ɢ�Ѱ�������Ȼ��ʹ�������Ž���Ϊ���Ҫ������������������ܹ�ʹ��multiplexed immunoassay����ͬʱ����������Ѱ��ʼ�������ת���IJ�Ʒ�������Ա�������ֹ�Ч����б���������
��������ëϸ��Western Blot�����������Ѱ���ҩ���PK�о���Ӧ����Ȼ����������Li������С���о�����֤����Ũ����PKҪ�������Զ���С��Ѫ���е�polyhistidine N-��FLAG C-terminally-tagged�����Ѱף�Լ55kDa��������LLOQΪ20ng/mL������Anti-FLAG tag������immunoblot�취���������������������о���������ֻ����1:100��1:500�ı���ϡ����Ʒ�����������������и�Ʒò�Ѱ�����������
�������Kodani����ʹ��capillary western blot�������ײ�����HCV����IgG����������ע���ڿ�ҩ��壨ADA����ⷽ���DZ��Ӧ������������HCV�Ѱ�������ëϸ������ɢ���ο�������ϡ�͵�����Ѫ�������ëϸ���з�������ʹ��HCV�������ο��Ŀ�ԭ�Ž���������һ�취֮������ʹ�ÿ�����IgG-HRP�����پ��еڶ��η�����������70���ر����������Ѫ�������ļ����������Ҫ������һ�����۵Ŀ�HCV�������Ҫ������������������
����
Ϊ��ʶ��˫�����Է��ӿ����������������������֧�����������������ڿ�����Щҩ����ӵ�PK����ս��ʱһЩ�����˼�����������������ս�Ժ�Ҫ�����Ӧ����˫�����Է��Ӻ�������������ҩ����������Ҫ���ض���ҩ����������û��ƣ�MOA����DZ�ڵİб�����ѧ�����Լ�����PK/PD������Ҫ����������ȫ����ʶ������
���������������ҵ��Ҫ�µĶ�����������������multiplexed PK����Ҫ�������Ա㶨���ش�ģʽ��ҩ�P������ת����Ʒ���������������Ѱ��������ģ�IC-��LC-HR-MS��capillary Western blot����ӵ�кܴ�Ӧ��Զ����������multiplexibility���ṩĿ�Ĵ�����Ķ�ά��Ϣ����������ȵ�����Ч/domain functionality������������������������������ HR-MS��capillary Western blot platforms�Ķ���Ѹ�ٶ�����������������ֻ����Щ��������Ҫ��һ�����������Ի�ø��ձ�Ľ���������Ŀ��HR-MSϵͳ��һ��ƽ��������Լ���������HR-MS���Լ�capillary Western blot��������������ڹ�ͺ���֤�������ϵ�ĽǶȿ���������Ҫ��һ����ȷ������
������ĺ�����׳־�ĽǶ�˼��������ý�PK/PD/����ԭ��/����ת���������������ϵ�һ����������Ҫ��֮����������̭ҩ�↑���������Դ������������ͳһ�黼����������ȫ����ҩ���PK/PD��Ϊ��������ƺͿ��������Ķ���������Ҫ��������ÿһ������ҩ�ṹ���������������Ҫһ�ֳ���“�ʺ�����;”��Ҫ�켰��ȷ�ϣ�confirmation��������Ϊ˫���ࣩ�����Է��ӿ����ɿ����Ƚ��Ϳ����ֵ�PK����Ҫ���Ǻ��Ǿ�����ս�Ե��������ڶ������ػ�Ӱ��ȷ��accurate���������壨meaningful����Ũ�Ȳⶨֵ������δ������������ҵ���ϵ������ָ������������Щ�߶��ش������Ѱף�ҩ��������������ۺ�����������Ἣ��ֵ������
δ��Զ��
������������Ҫ��������ԡ�ѡ���Ժ�Ѹ�ٶȵ�һֱ���������ȷ��accurate����ϸ�ܣ�precise���IJⶨ˫�����Կ���Ũ�Ⱥ���������������Ʒ�е�����ԭ�Խ���Ϊ����������Ԥ��˫�����ԺͶ�����������ҩ����Ŀ�����ཫ�������������⽫�����µ���������Ҫ��Ŀ���������˳Ӧ��Щ���ӵĽṹ�ش��Ժ�MOAs������
��Ȼ�������ڵ�Ҫ�콫�������µ��Ѱ�ҩ��Ķ���������ֻ��LBAҪ����������������Ҫ�����Ҫѡ�����������ڿ���Ԥ����δ���Խ���������LC-MS/MSҪ����������������������multiplexed�Ķ������������������ܻ���Խ���ձ��Ӧ��������֧��˫����������ҩ��PK����������LC-MS/MS��LBA�������и��ٵ����������ԣ�variability����Ҫ���Լ��Ŀɼ��ԣ�availability������������Ԥ������ʵ���ҵ��Զ�������չ���������������н���������̭�˹���ʧ�������������������
��������
����������©��������ָ�Ϻ����ݵĵط����������̸�ۺ�ָ���������������õ�ԭʼ��Ϣ�����Ͼ������Ѿ�����ѧ���ڿ����ٷ����籨���ȹ�������, ���漰�κα�����Ϣ�������ο�����ѡ��˼������������Ҳ������������������Ӵ������ṩ�м�ֵ������������������
�� �� �� ��
1.Zhu, L, et al. (2020). "Bioanalytical Challenges in Support of Complex Modalities of Antibody-Based Therapeutics." AAPS J 22(6): 130.
2.Ma, M., et al. (2019). "Bioanalytical challenges and unique considerations to support pharmacokinetic characterization of bispecific biotherapeutics." Bioanalysis 11(5): 427-435.
3.Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab). J. Cancer 2, 309–316 (2011).
4.Mullard A. Bispecific antibody pipeline moves beyond oncology. Nat. Rev. Drug Discov. 16(11), 666–668 (2017).
5.Diao L, Meibohm B. Tools for predicting the PK/PD of therapeutic proteins. Expert Opin. Drug Metab. Toxicol. 11(7), 1115–1125 (2015).
6.Trivedi A, et al. Clinical pharmacology and translational aspects of bispecific antibodies. Clin. Transl. Sci. 10(3), 147–162 (2017).
7.Ezan E, et al. Assessment of the metabolism of therapeutic proteins and antibodies. Expert Opin. Drug Metab. Toxicol.10(8), 1079–1091 (2014).
8.Fischer SK, et al. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters. Case studies. MAbs 4(5), 623–631 (2012).
9.Ruf P, et al. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br. J. Clin. Pharmacol. 69(6), 617–625 (2010).
10.Samineni D, et al. Impact of shed/soluble targets on the PK/PD of approved therapeutic monoclonal antibodies. Exp. Rev. Clin. Pharm. 9(12), 1557–1569 (2016).
11.Villegas VM, et al. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin. Drug Deliv. 14(2), 273–282 (2017).
12.Ruppel J, et al. Preexisting antibodies to an F(ab’)2 antibody therapeutic and novel method for immunogenicity assessment. J. Immunol. Res. 2016, 1–8 (2016).
13.Fan X, et al. Lens glutathione homeostasis: discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 156, 103–111 (2017).
14.Kang L, et al. LC-MS bioanalysis of intact proteins and peptides. Biomed Chromatogr. 2020;34(1):e4633. https://doi.org/10.1002/bmc.4633.
15.Chen, J, et al. "Capillary nano-immunoassays: advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics." Journal of translational medicine 13: 182. (2015)
16.Murphy RE, et al. Combined use of immunoassay and twodimensional liquid chromatography mass spectrometry for the detection and identification of metabolites from biotherapeutic pharmacokinetic samples. J Pharmaceut Biomed.2010;53(3):221–7. https://doi.org/10.1016/j.jpba.2010.04.028.
17.He JT, et al. High resolution accurate-mass mass spectrometry enabling in-depth characterization of in vivo biotransformations for intact antibody-drug conjugates. Anal Chem. 2017;89(10):5476–83.https://doi.org/10.1021/acs.analchem.7b00408.
18.Jian WY, et al. A workflow for absolute quantitation of large therapeutic proteins in biological samples at intact level using LC-HRMS. Bioanalysis.2016;8(16):1679–91. https://doi.org/10.4155/bio-2016-0096.
19.Lanshoeft C, et al. Generic hybrid ligand binding assay liquid chromatography high resolution mass spectrometry based workflow for multiplexed human immunoglobulin G1 quantification at the intact protein level: application to preclinical pharmacokinetic studies. Anal Chem. 2017;89(4):2628–35. https://doi.org/10.1021/acs.analchem.6b04997.
20.Jin W, et al. LC-HRMS quantitation of intact antibody drug conjugate trastuzumab emtansine from rat plasma. Bioanalysis. 2018;10(11):851–62. https://doi.org/10.4155/bio-2018-0003.
21.Zhang LY, et al. Top-down LC-MS quantitation of intact denatured and native monoclonal antibodies in biological samples. Bioanalysis. 2018;10(13):1039–54. https://doi.org/10.4155/bio-2017-0282.
22.Li Y, et al. An efficient and quantitative assay for epitope-tagged therapeutic protein development with a capillary western system. Bioanalysis. 2019;11(6):471–84. https://doi.org/10.4155/bio-2018-0248.
23.Kodani M, et al. An automated immunoblot method for detection of IgG antibodies to hepatitis C virus: a potential supplemental antibody confirmatory assay. J Clin Microbiol. 2019;57(3). https://doi.org/10.1128/JCM.01567-18.
������2��������¼���ҽҩ �ٴ��о�����
��2��������¼���ҽҩӵ��һ֧��ģ�ش�רҵ������ٴ��о������������ṩ����ҽѧ����Ŀ��������顢���졢���ݹ�����ͳ����������������������ڵ��ٴ�����ȫ���̽���ƻ���������ֹ2020��������2��������¼���ҽҩ����Ŀͻ���1000���������800�����ٴ�������Ŀ���������ͻ������ҩ֤��60��������������80��������ӵ�и�����ٴ����������������������Ŀ�����ٴ��о����������������������β�������������ҩ����ӵ��������ٴ�����ϵͳ������
��2��������¼���ҽҩ����������40����ٴ�����������������½�600���ٴ��������������������������ORACLE OC/RDC��CTMSϵͳ���������ٴ���������ʵʱ�ԡ������ٴ��������̵Ĺ淶��������































